Abstract
Effective immunotherapy for acute myeloid leukemia (AML) has been limited by the lack of AML-restricted targets (expression in AML but silent in normal hematopoiesis). Current immunotherapies targeting lineage markers CD33 and CD123 (if effective) would lead to prolonged myelosuppression or myeloablation, requiring stem cell transplantation to restore hematopoiesis after treatment. In search for AML-restricted targets, we interrogated the AML transcriptome of nearly 3000 cases in pediatric and young adults and contrasting it to normal bone marrow and peripheral blood CD34+ samples. This extensive discovery effort has identified over 200 AML-restricted targets with mesothelin (MSLN) emerged as one of the highest expressing AML-restricted targets and highly enriched in the KMT2A-rearranged AML subtype. We previously validated cell surface expression of MSLN on both AML blasts and leukemic stem cells (Le et. al. 2021). We further showed efficacy targeting MSLN in AML using antibody-drug conjugates (Kaeding et. al. 2021) and chimeric antigen receptor (CAR) T cells (Le et. al. 2021). Given that natural killer (NK) cells are potent immune effector cells and generally have a more favorable toxicity profile than CAR T cells (i.e without cytokine release syndrome), we developed CAR NK cells targeting MSLN and evaluated their efficacy in AML preclinical models.
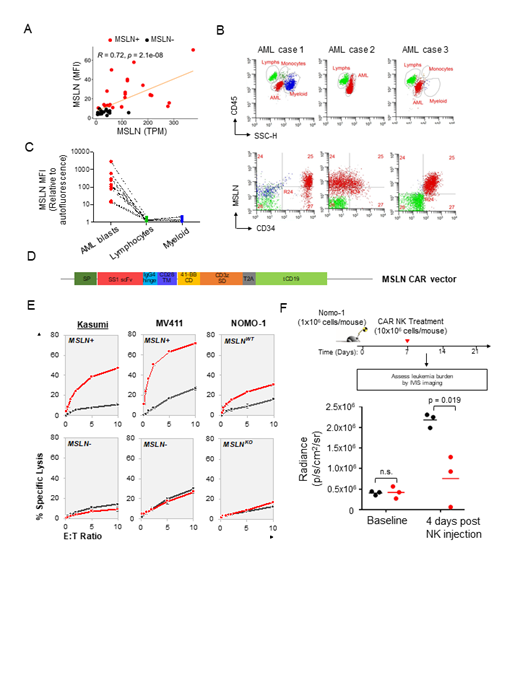
From primary patient samples, we verified MSLN cell surface expression and showed high correlation between cell surface expression (mean fluorescence intensity, MFI) and transcript expression (TPM, R = 0.72, p = 2.1x10 -8, Figure 1A) . Importantly, MSLN expression was restricted to AML blasts and entirely absent in normal lymphocytes and myeloid cells in individual patients (Figure 1B, C). Having confirmed cell surface and AML-restricted expression of MSLN, we developed CAR NK cells targeting MSLN. The VH and VL sequences from immunotoxin SS1P were used to create the single-chain variable fragment domain of the standard CAR (41-BB and CD3Zeta, Figure 1D). CAR NK cells were generated by transducing NK-92 cells with the MSLN CAR construct. Cytotoxicity of CAR NK cells was evaluated against Nomo-1 AML cell line, which expresses endogenous level of MSLN; MV4;11 and Kasumi-1 cell lines engineered to express MSLN with a lentivirus construct (MV4;11 MSLN+ and Kasumi-1 MSLN+).
We initially tested the target specificity of MSLN-directed CAR NK cells against MSLN-positive (Nomo-1, MV4;11 MSLN+ and Kasumi-1 MSLN+) and MSLN-negative (Nomo-1 MSLN KO, MV4;11 and Kasumi-1) cells. CAR NK cells exhibited enhanced cytolytic activity against MSLN-positive but not MSLN-negative AML cells after 12 hours of co-incubation at the indicated effector: target (E:T) ratios (Figure 1E). We next assessed the in vivo efficacy of CAR NK cells. Nomo-1 cells transduced with GFP/Luciferase were transplanted into NSG mice at 1x10 6 cells/mouse. Unmodified or CAR NK cells were infused 1 week following leukemic cell injection at 1x10 7 cells/mouse. Monitoring leukemia burden by bioluminescence IVIS imaging showed that after 4 days post NK injection, the leukemia was significantly reduced in Nomo-1 xenograft mice treated with CAR NK cells compared to mice that received unmodified NK cells (Figure 1F), suggesting highly potent anti-leukemia activity of CAR NK cells.
In this study, we demonstrate that the cell surface expression of MSLN is restricted to AML blasts but is entirely silent in normal hematopoietic subsets in individual patients. Previous and current clinical trials utilizing NK-92 cells have demonstrated safety and efficacy in variety of cancers, including AML. Here, we demonstrate that NK-92 cells genetically modified with a CAR to redirect their specificity against MSLN-positive AML cells exhibit potent, target-dependent anti-leukemia activity in vitro and in vivo. These results provide compelling data to evaluate MSLN-directed CAR NK cell therapy in clinical trials for refractory/relapsed AML, especially for high-risk KMT2A-rearranged leukemia where majority of patients express MSLN at diagnosis and relapse.
Pardo: Hematologics, Inc.: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal